Audi Q3: A/C Technology Basic Principles
Physical Principles
The four known states of water also apply to air conditioning system refrigerants.
1 - Gaseous (invisible)
2 - Vapor
3 - Liquid
4 - Solid
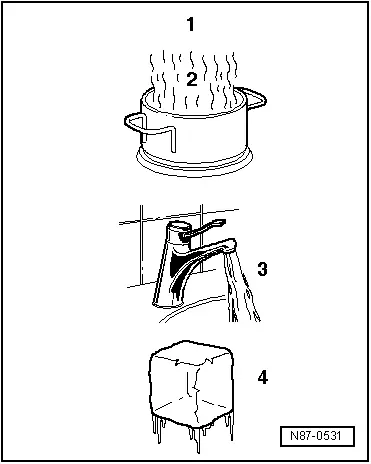
When water is heated in a container (heat absorption), water vapor can be seen to rise. If the vapor is further heated through heat absorption, the visible vapor turns into invisible gas. The process is reversible. If heat is extracted from water in gaseous form -A-, it changes first to vapor -B-, then to water and finally to ice.
A - Heat absorption
B - Heat emission
Heat Transfer
Every substance consists of a mass of moving molecules. The fast moving molecules of a warmer substance give off some of their energy to the cooler and thus slower molecules. As a result, the molecular motion of the warmer substance slows down and that of the colder substance is accelerated. This process continues until the molecules of both substances are moving at the same speed. They are then at the same temperature and no further heat exchange takes place.
Pressure and Boiling Point
The boiling point given in tables for a liquid is always referenced to an atmospheric pressure of 1 bar (14.5 psi). If the pressure acting on a fluid changes, its boiling point also changes.
 Note
Note
Pressure is measured in different units: 1 MPa (145 psi) corresponds to 10 bar (145 psi) positive pressure. 1 bar (14.5 psi) absolute pressure corresponds to 0 bar/psi positive pressure and thus to the ambient pressure (atmospheric pressure).
Water boils at a lower temperature the lower the pressure.
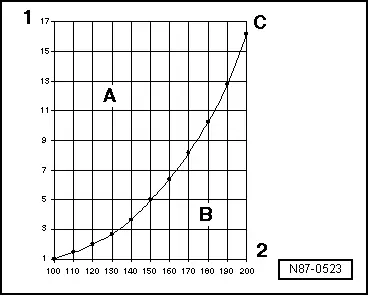
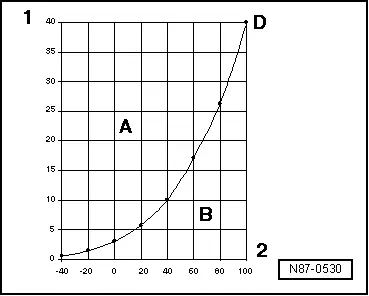
The vapor pressure curves for water and refrigerant R134a show that, at constant pressure, reducing the temperature changes vapor to liquid (in the condenser) or that reducing the pressure causes the refrigerant to change from liquid to vapor (inside the evaporator).
Vapor pressure curve of water
A - Liquid
B - Gas
C - Vapor pressure curve of water
1 - Pressure acting on liquid in bar (psi)
2 - Temperature in ºC (ºF)
Vapor pressure curve of refrigerant R134a
A - Liquid
B - Gas
D - Vapor pressure curve of refrigerant R134a
1 - Pressure acting on liquid in bar (psi)
2 - Temperature in ºC (ºF)
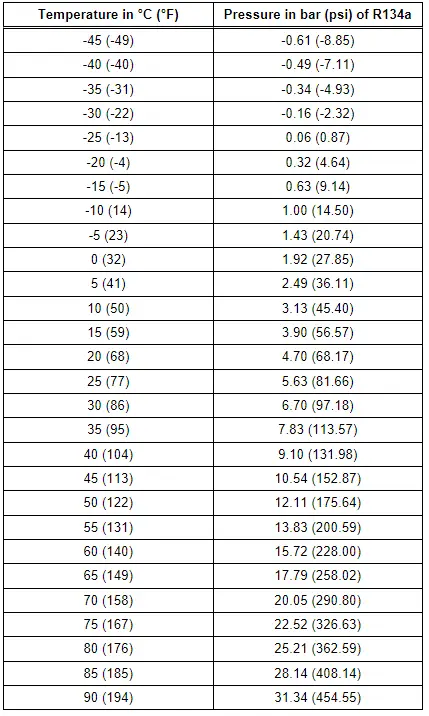
Refrigerant R134a Vapor Pressure Table
The vapor pressure table for every refrigerant is published in literature for the refrigeration system engineers. This table makes it possible to determine the vapor pressure acting on the column of liquid in a container if the temperature of the container is known.
Because each refrigerant has its own characteristic vapor pressure table, refrigerant can be identified by measuring the pressure and temperature.
 Note
Note
- At absolute pressure, "0 bar/psi" corresponds to absolute vacuum. Normal ambient pressure (positive pressure) equals approximately "1 bar (1.5 psi)" absolute pressure. "0 bar/psi" pressure corresponds to an absolute pressure of 1 bar (14.5 psi) on most pressure gauges (indicated by "-1 bar (-14.5 psi)" below "0 bar/psi").
- Pressure is measured in different units: 1 MPa (145 psi) corresponds to 10 bar (145 psi) positive pressure. 1 bar (14.5 psi) absolute pressure corresponds to 0 bar/psi positive pressure and thus to the ambient pressure (atmospheric pressure).
Refrigerant R134a
Refrigerant R134a Physical Data
Vehicle air conditioning systems make use of the vaporization and condensation process. In this case, one works with a substance which boils easily, designated as refrigerant.
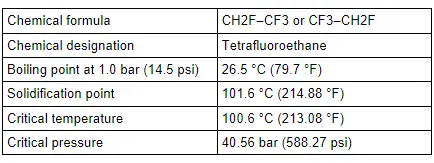
The refrigerant used is tetrafluoroethane R134a, which boils at -26.5 ºC (-15.7 ºF) at a vapor pressure of "1 bar (14.5 psi)" (absolute pressure corresponds slightly to the ambient pressure).
Critical Point
The critical point (critical temperature and critical pressure) is that above which there is no longer a boundary between liquid and gas.
A substance above its critical point is always in the gaseous state.
At temperatures below the critical point, all types of refrigerant in pressure containers exhibit both a liquid and a gas phase, for example there is a layer of gas above the liquid.
As long as both liquid and gas are present in the container, the pressure is governed by ambient temperature. Refer to → Chapter "Refrigerant R134a Vapor Pressure Table "vapor pressure table".
 Note
Note
Different types of refrigerant are never to be mixed. Only the refrigerant designated for the corresponding A/C system may be used.
Refrigerant R134a Environmental Information
- R134a is a fluorocarbon and contains no chlorine.
- R134a has a shorter atmospheric life span than refrigerant R12.
- R134a does not damage the ozone layer. The ozone depletion potential is zero.
- The greenhouse potential of R134a (Global Warming Potential = GWP) is approximately 1430 (the GWP of carbon dioxide = 1). To reduce the influence of the greenhouse effect of the refrigerant R134a, the European commission has committed that a vehicle from the 01/01/2017 with refrigerant in the vehicle A/C system with a GWP greater than 150 can no longer be placed on the market. A/C system in vehicles which were brought into the market through 12/31/2016 can until further notice be filled with refrigerant R134a and driven.
- The global warming effect of R134a is "10" times less than that of refrigerant R12.
Refrigerant R134a Characteristics
Commercial Names and Designations
Refrigerant R134a is currently available under the following trade names:
- H-FKW 134a
- SUVA 134a
- KLEA 134a
 Note
Note
- Different trade names may be used in other countries.
- Of the wide range of refrigerants available, this is the only one which may be used for vehicles. The designations Frigen and Freon are trade names. They also apply to refrigerants which may not be used in automotive vehicles.
Color
Like water, refrigerants are colorless in both vapor and liquid form. Gas is invisible. Only the boundary layer between gas and liquid is visible. (Liquid level in tube of charging cylinder or bubbles in sight glass). Refrigerant R134a fluid may appear colored (milky) in a sight glass. This cloudiness is caused by partially dissolved refrigerant oil and does not indicate a malfunction.
Vapor Pressure
In a partially filled, closed container, the quantity of refrigerant evaporating from the surface equals the quantity returning to the liquid state as vapor particles condense. This state of equilibrium occurs under the influence of pressure and is often called vapor pressure. The vapor pressure is dependent on the temperature. Refer to → Chapter "Refrigerant R134a Vapor Pressure Table "vapor pressure table".
R134a Physical Characteristics
The vapor pressure curves of R134a and other refrigerants are sometimes very similar, therefore it is not possible to make a certain distinction solely by pressure.
With R134a, the A/C compressor is lubricated with special synthetic refrigerant oils, for example, PAG oils (polyalkylene glycol oils).
Affect on Metal
In its pure state, refrigerant R134a is chemically stable and does not corrode iron or aluminum.
Refrigerant impurities such as chlorine compounds however cause corrosion of certain metals and plastics. This can lead to blockage, leaks or deposits on the A/C compressor piston.
General Information
The refrigerant R134a remains chemically stable up to a gas pressure of 39.5 bar (573 psi) (absolute pressure of 40.56 bar (588 psi), which corresponds to a temperature of 101 ºC (214 ºF) ). Above this temperature, the refrigerant decomposes.
Water Content
Only very small amounts of water are soluble in liquid refrigerant. On the other hand, refrigerant vapor and water vapor mix in any ratio.
Any water in the refrigerant circuit will be entrained in droplet form once the dryer in the receiver or reservoir has absorbed as little as approximately 7g of water. This water flows as far as the nozzle of the expansion valve or restrictor and turns to ice, the A/C system no longer has a cooling effect.
Water destroys the air conditioner as it combines with other impurities at high pressures and temperatures to form acids.
Combustibility
Refrigerant is non-flammable. It actually has a fire-resistant or fire extinguishing effect. Refrigerant decomposes when exposed to flames or red-hot surfaces. UV light (occurring for example during electric welding) also causes refrigerant decomposition. The resultant decomposition products are toxic and are not to be inhaled. However, irritation of the mucous membranes provides an adequate and timely warning.
Charge Factor
A container must have space for vapor as well as liquid. As the temperature rises, the liquid expands. The space filled with vapor decreases. At a certain point, there will only be liquid in the container. Beyond this, even a slight increase in temperature causes high pressure to build up in the container as the liquid tries to continue expanding even though there is not enough space for it. The forces that result are strong enough to rupture the container. To prevent a container from being overfilled, the regulations regarding compresses gasses specify how many kilograms of refrigerant that may be added to a container per liter of interior volume. The product of multiplying this charge factor by the internal volume of the container is the permissible capacity. The figure for refrigerant used in vehicles is 1.15 kg/liter.
Evidence of Leaks
External damage, for example, can cause a leak in the refrigerant circuit. The small quantity of refrigerant escaping from minor leaks can be detected, for example, by using an electronic leak detector or by introducing a UV-leak detection additive into the refrigerant circuit. Electronic leak detectors are capable of registering leaks with refrigerant losses of less than 5g per year.
 Note
Note
Use leak detectors designed for the type of refrigerant. For example, a leak detector for R12 refrigerant will not work with R134a because R134a refrigerant has no chlorine atoms so the leak detector will not respond to it.

